The United Kingdom on Wednesday authorized the emergency use of the Covid-19 vaccine developed by Pfizer and BioNTech.
It makes the UK the first country to approve the vaccine which is developed for adults age 16 and older.
The UK has ordered 40 million doses of the vaccine — enough to vaccinate 20 million people.
“I’m confident now, with the news today, that from spring, from Easter onward, things are going to be better and we’re going to have summer next year that everyone can enjoy,” said UK Health Secretary Matt Hancock.
UK’s Medicines and Healthcare Products Regulatory Agency (MHRA) granted the temporary authorization shortly after Pfizer and BioNTech reported in November that the Covid-19 vaccine was 95 percent effective.
Prime Minister Boris Johnson called the news “fantastic” in a Downing Street press conference on Wednesday.
Joint Committee on Vaccination and Immunisation (JCVI) advises that the first priorities for the COVID-19 vaccination programme should be the prevention of mortality and the maintenance of the health and social care systems. As the risk of mortality from COVID-19 increases with age, prioritisation is primarily based on age. The order of priority for each group in the population corresponds with data on the number of individuals who would need to be vaccinated to prevent one death, estimated from UK data obtained from March to June 2020. For this reason, starting next week, around 50 “hospital hubs” will begin offering the vaccine to people over 80 and care home staff.
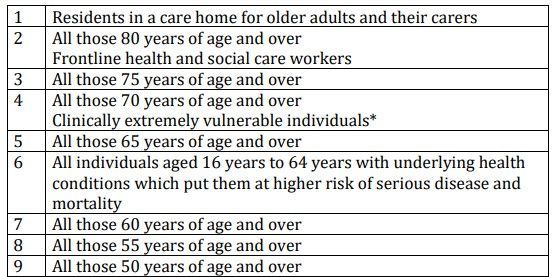
* Clinically extremely vulnerable individuals are described here. This advice on vaccination does not include pregnant women and those under the age of 16 years
Meanwhile, in the United States, the U.S. Food and Drug Administration has scheduled a meeting of its Vaccines and Related Biological Products Advisory Committee (VRBPAC) on December 17 to discuss the request for emergency use authorization (EUA) for a COVID-19 vaccine from Moderna Inc.
A panel of outside advisers to the F.D.A. is scheduled to meet on December 10 to decide whether the agency should grant emergency authorization to the Pfizer vaccine.
An independent panel advising the Centers for Disease Control and Prevention voted Tuesday to recommend that residents and employees of nursing homes and similar facilities be the first people in the United States to receive coronavirus vaccines, along with health care workers who are especially at risk of being exposed to the virus.
“States are not required to follow the panel’s recommendations, but they usually do,” says Dr. Beth Bell, a panel member and global health expert at the University of Washington.
Pfizer and Moderna have estimated that they will have enough to vaccinate, at most, 22.5 million Americans by year’s end, with the required two doses, a few weeks apart. The C.D.C. will apportion the supply among the states, with the initial allocation proportional to the size of each state’s adult population, according to the New York Times.