In a critical move to safeguard the health and security of millions, the Director General of Africa Centres for Disease Control and Prevention (AfricaCDC) has officially declared the ongoing mpox outbreak a Public Health Emergency of Continental Security (PHECS).
This urgent declaration, made during a special press briefing, underscores the severity of the crisis and the immediate need for coordinated action across Africa.
As Africa grapples with a continent-wide public health emergency, the European Union (EU) has responded by sending 175,420 doses of vaccines to the Africa Centres for Disease Control and Prevention (AfricaCDC) to combat the rising cases of mpox, previously known as monkeypox. This urgent measure, announced by the European Commission, highlights the international collaboration required to address the escalating outbreak.
The AfricaCDC’s declaration of mpox as a public health emergency underscores the severity of the situation. With a target of securing two million vaccines, the AfricaCDC’s call for international assistance has been met with prompt action from the EU. The vaccines, delivered through the EU’s Health Emergency Preparedness and Response Authority (HERA), are expected to play a crucial role in containing the spread of the viral disease across the continent.
What is Mpox?
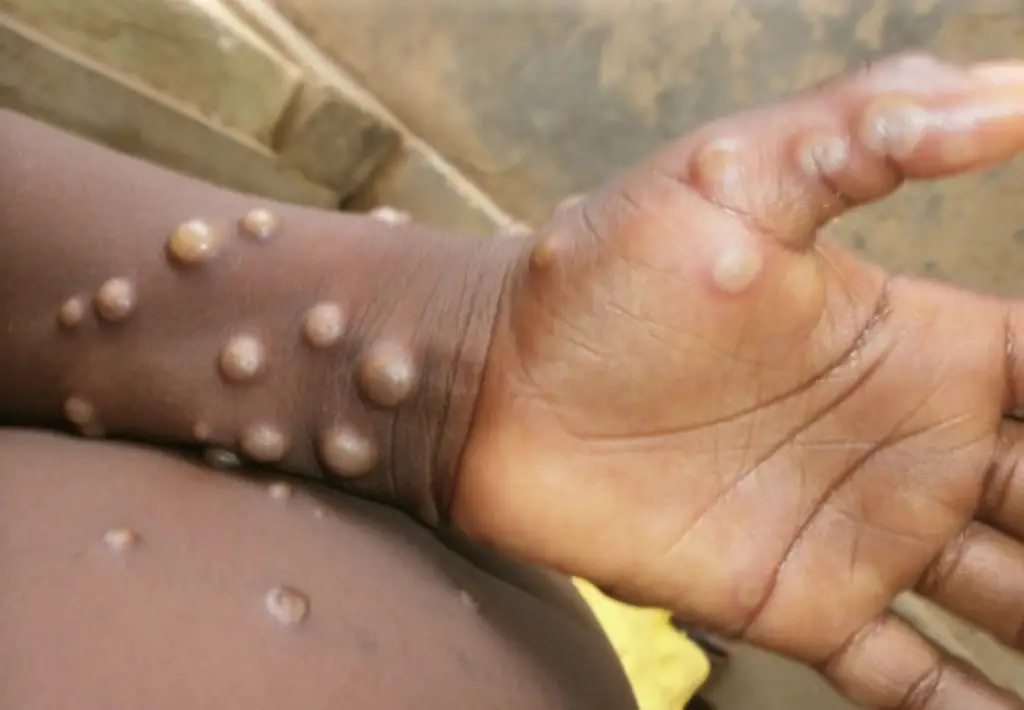
Mpox is a viral infection that causes a blistering rash, is transmitted through physical contact with infected individuals, animals, or contaminated materials.
Symptoms of mpox typically appear within 5 to 21 days of exposure and include fever, headache, muscle aches, back pain, and swollen lymph nodes, followed by the development of a distinctive rash. The rash often begins on the face and spreads to other parts of the body, including the palms of the hands and the soles of the feet. The blisters eventually crust over and fall off as the patient recovers.
While most individuals with mpox fully recover, the disease can pose serious health risks, particularly in populations with compromised immune systems or in regions with limited access to healthcare. The current outbreak has raised significant concern due to its rapid spread and the potential for severe complications in vulnerable groups, making it a critical focus for public health authorities worldwide.
International Response and Vaccine Distribution
In addition to the EU’s contribution, pharmaceutical company Bavarian Nordic has pledged to donate 40,000 doses of its Modified Vaccinia Ankara-Bavarian Nordic (MVA-BN) vaccine to HERA. This vaccine is currently approved for use in at-risk adults in the EU, the United Kingdom, and the United States, among other regions. However, only two African countries have approved its use so far, prompting the World Health Organisation (WHO) to urge Bavarian Nordic to seek Emergency Use Listing to expedite its availability in the affected areas.
The WHO’s Emergency Committee convened in Geneva to discuss the outbreak and consider whether to declare a Public Health Emergency of International Concern (PHEIC). The decision is still pending, reflecting the global concern over the spread of mpox and the need for coordinated international efforts.
Urgent Need for Global Cooperation
The EU’s vaccine shipment to Africa is a critical step in supporting the continent’s response to the mpox outbreak. As the situation continues to evolve, the collaboration between international organizations, governments, and pharmaceutical companies remains vital. The distribution of vaccines is just one aspect of a broader strategy needed to curb the outbreak and protect vulnerable populations.
The global community’s response to the mpox crisis serves as a reminder of the importance of preparedness and cooperation in addressing emerging health threats. With timely intervention and sustained support, there is hope that the outbreak can be brought under control, safeguarding public health across Africa and beyond.
Photo credit: Nigeria Centre for Disease Control